Good disease management is critical to the successful production of the white potato (Solanum tuberosum L.). The potato plant is susceptible to at least 75 diseases and nonparasitic disorders, many of which consistently cause yield losses in potato production areas in the northeastern United States. Potatoes are a vegetatively propagated crop, and potato seed tubers can be an important source of disease inoculum. Several types of injury that can influence plant vigor and subsequent yields also occur on seed tubers. The management of many production problems depends on the identification of diseased or defective seed tubers.
The objective of this factsheetis to aid in the diagnosis of those tuber diseases and defects that most often result in production problems. Twenty selected diseases and defects that affect potato tubers and are most important to potato production in the northeastern United States are discussed. Symptoms of these diseases and disorders, as they appear on the tubers, are described and illustrated.
The Why, When, and How of Potato Seed Evaluation
Why
Most of the tuber defects described, when present on seed, can cause substantial reductions in yield or quality in the subsequent crop under the right environmental conditions. However, some tuber problems are much more likely to cause significant losses than others and, consequently, serve to answer the question of why potato seed evaluation is important. Diseases, such as ring rot, late blight, and leafroll (net necrosis), that are carried on or in the seed and have the potential to spread very quickly through the crop are considered to be very important. Tubers infected with such diseases must be properly discarded as soon as they are detected. Other diseases, such as Rhizoctonia black scurf and Pythium leak, that do not have significant secondary spread and for which other sources of inoculum are usually more important are considered to be less serious. However, remember that the absolute losses resulting from specific tuber problems will depend upon environmental conditions and disease management practices. The most important aspect of disease management in potato production is the use of certified seed potatoes. There are two basic classes of seed potatoes–foundation and certified. Requirements for the production of foundation class are much more rigid. Another important aspect of disease management in potato tubers is the control of disease spread in storage. Many diseases can increase significantly during the storage period, and management of the environmental conditions in the potato storage is often a critical component of control. A summary of some important aspects of the 20 diseases and defects discussed in this factsheet can be found in the table, Summary of important aspects of 20 potato diseases and defects.
When
Although most of the disorders discussed can be detected just before planting, many can also be identified in seed production fields or in storage facilities. Early detection of seed disorders provides the seed grower with additional management and storage options. It provides the seed purchaser with knowledge of the condition of the seed acquired. Shipping point inspection, the final step in certification, cannot be overemphasized. It is paid for by the grower and serves as protection for both grower and buyer. It should be kept in mind that certification is not complete until the seed has been graded in conformity to certified seed grades and identified with official tags and has passed inspection by the State–Federal Inspection Service. If a problem should arise as to seed quality once the tubers are delivered to their final destination, the buyer may request a second inspection, at his or her expense, to determine if the seed lot is in compliance with the stated grades. Without inspections and shipping tags, the old adage still applies, “Let the buyer beware.” Figure 1 illustrates a generalized picture of potato production in the Northeast and indicates the stages during which specific diseases and disorders affect potato production and storage and when they are most apt to be detected.
How
The 20 disorders described have been grouped into three categories, based on the location of tuber symptoms:
- External: Four of the diseases and defects are diagnosed entirely from symptoms that occur on the exterior of the tuber. The position of the symptoms on the surface of the tubers, relative to the stem attachment, bud end, and lateral eyes, is often important in diagnosis.
- Internal:Five of these disorders usually produce no diagnostic symptoms on the exterior of the tuber, and diagnosis must be based solely on internal symptoms. Specific tissues in the interior of the tuber must often be examined for symptoms. Therefore, either cross–sectional or longitudinal cuts must be made through the tuber to observe symptoms.
- External/Internal: Eleven tuber disorders discussed here usually require examination of both external and internal tuber symptoms for diagnosis. Although the symptoms are noted externally, it is best that tubers be cut to determine to what depth the lesion has progressed. This is particularly helpful for early and late blight. Information on the location of tuber symptoms for each of the 20 disorders has been summarized in the table, Summary of important aspects of 20 potato diseases and defects.
Many excellent references are available on potato disease control; but because some control recommendations change with time, only minimal information on this subject is included. Information on control, primarily with cultural practices, is included in the description of the individual disorders. For labeled fungicides see current Cornell Integrated Crop and Pest Management Guidelines for Commercial Vegetable Production.
External Diagnosis
Common scab – Streptomyces scabies (bacterium)
Common scab is often characterized by a corkiness of the tuber periderm, but symptoms are extremely variable. Lesions may be very superficial or may penetrate up to 1/4 inch into the tuber surface and have been described as russetted, slightly raised, slightly pitted, or deeply pitted. Up to 100 percent of the tuber surface may be affected by lesions, which are light brown to dark brown. Insects are attracted to affected tissue and may enlarge lesions. Symptoms of common scab may be confused with those of powdery scab, Rhizoctonia russeting, or insect damage. This disease usually occurs in soils with pH values higher than 5.2 and when the soil is dry during tuberization. Acid–tolerant Streptomyces spp. that cause symptoms indistinguishable from S. scabies have been reported. Although common scab does not spread in storage, infected seed can lead to infection of daughter tubers and contamination of soil. Avoid heavy manuring, especially with poultry manure. Controls include clean, certified seed, resistant varieties, and fungicide seed treatments that are active against S. scabies.

Powdery scab – Spongospora subterranea (fungus)
Immature lesions occur on the tuber surface and are white and wartlike, then gradually become darker. Mature lesions appear as open pustules, which contain olive brown to black powdery masses of spores, often surrounded by the remnants of the periderm. The presence of the granular–appearing spores in mature lesions is diagnostic, but they may require magnification for positive identification. Lesions are not corky in appearance as are those of common scab, but may be confused with this very different disease. Powdery scab is favored by heavy soils and high soil moisture. This disease can spread in storage or lead to dry rot. Inoculum from infected seed tubers can spread to daughter tubers and cause infection. Clean seed and long rotations are recommended for control of powdery scab.
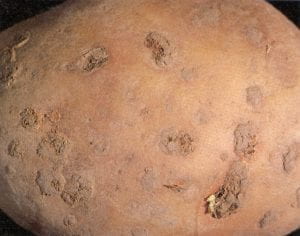
Rhizoctonia (black scurf) – Rhizoctonia solani (fungus)
This fungus is a common soil inhabitant and tuber colonizer, most easily identified by the small black sclerotia (black scurf stage) on the tuber surface. Sclerotia range in size from as small as a pinhead to as large as half a pea and are best described as dirt that won’t wash off, but can be removed with a fingernail. Rhizoctonia also causes a russeting or checking of the tuber surface, which is common in heavier soils. These symptoms can be confused with those of common scab. The sclerotia remain inactive in storage, but contribute to poor appearance and reduced marketability. Tubers used for seed should be treated with a fungicide, for sclerotia can be a source of inoculum for sprout “burn” and stem cankers on early emerging plants. Delayed harvest increases sclerotia size and number.

Silver scurf – Helminthosporium solani (fungus)
Silver scurf is relatively common on tubers, including those grown for seed. However, the symptoms of this disease may go unnoticed unless tubers are very carefully examined. A fine coating of dark green to black spores, visible to the naked eye only in mass, can sometimes be seen on the surface of infected tubers that have been stored under conditions of high humidity. However, the best way to detect this disease is by washing the tubers and looking for a silvery sheen that occurs in patches on the tuber surface. These patches may cover a large portion of the tuber and are caused by air space, which results from growth of the fungus beneath the tuber periderm. Symptoms can be difficult to detect on some white–skinned cultivars, but are obvious on those with red skin. Heavily infected tubers may not sprout properly and are an important source of inoculum for subsequent infection of daughter tubers. Infection does spread in storage, but can be limited by maintaining temperatures of 40° F and providing forced–air ventilation.


External/Internal Diagnosis
Bacterial soft rot – Erwinia carotovora subsp. carotovora(Ecc) and other bacteria
Soft rot is a very common, complex, and important disease of potato tubers. It is felt that much of the rot that develops in transit and storage is initiated in the field. In addition to E. carotovora subsp. carotovora (Ecc), two other Ercuinias can attack potato and cause soft rot; these include the blackleg organism E. carotovora subsp. atroseptica (Eca) and, in some geographical areas, E. chrysanthemi (Echr). The temperature at which diseased plant material is incubated can influence which soft rot bacteria are isolated. As temperatures increase from 61 ° to 99° F, the dominant populations shift from Eca to Ecc to Echr if all three bacteria are present initially. These pathogens can invade through injuries and enlarged lenticels. The first external symptoms are tan or watersoaked spots on the tuber surface, which eventually become slimy. Lenticel infection can result in raised dark brown lesions up to 1/a inch in diameter. Following entry of the bacteria, the tuber flesh becomes soft and rotten. The cream–colored rotted tissue may be separated from healthy tissue by a brown or black border.
Losses to soft rot can be reduced by avoiding injuries. Under optimum conditions suberization (protective tissue) develops within 24 to 48 hours after an injury. However, at temperatures below 50° F and above 95° F, healing proceeds at too slow a rate to provide much protection against wound pathogens. Because of this, it is best to hold newly harvested potatoes at moderate temperatures (60°–70° F) and at as high a humidity as possible for a period of 7–10 days before storage at low temperature (39° F). The seed should be warmed (at or near room temperature) before planting, and whenever possible, it should be planted in soil with a minimum temperature of 50° F at a 5–6–inch depth.

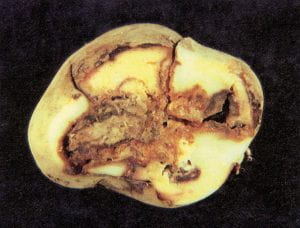
Blackleg– Erwinia carotovora subsp. atroseptica (Eca) (bacteria)
The pathogen can survive in soil as well as seed; however, planting infected seed pieces into wet, cold soils under anaerobic conditions results in the most severe losses in stand and yield. The bacteria enter the tubers through stolons during the growing season, with black sunken lesions resulting at the stem end. Infection normally spreads from the stem ends through the heart of the tuber. Internally the infected tuber flesh first appears cream colored, then turns grayish and black with a mushy texture like bacterial soft rot. Typically, infected tubers have soft rot only in the pith region, but in an advanced stage secondary soft rot bacteria may predominate (Ecc and Echr) and confuse the diagnosis. Control in the field relies upon disease–free seed that is adequately warmed and sprouted, and planted in soil with a minimum temperature of 50° F at a 5–6–inch depth. Rotations of 2–3 years between potato crops may also help.
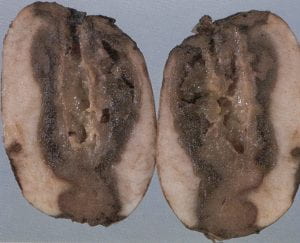
Early blight – Alternaria solani (fungus)
Tubers are not generally attacked by this fungus, but infection can occur when tubers become inoculated with spores before and during harvest. Lesions on the exterior of tubers are irregular in shape and range from 1/4 to 2 inches in diameter. These brownish black spots may be slightly sunken (approx. 1 / 16 in. deep) and have a raised purplish border. When tubers are cut through the lesions, they appear as a dry, dark brown rot, which is generally much shallower than that of late blight. Lesions are sharply set off from healthy tissue by a corky layer. Storing tubers below 41 ° F will stop further development of decay, and forced–air ventilation will reduce the chance of spread in storage. Foliar fungicide applications should be made when the disease first appears on leaves. Good vine killing, with a delay of a minimum of 7 days before digging, will also reduce the opportunity for tuber infection.

Freezing and chilling injury (nonpathogenic)
Symptoms are somewhat variable depending on the temperature, exposure period, and potato cultivar. Externally, the tuber appears wrinkled and flabby, whereas internal tissues generally turn bluish grey to black in response to cold temperatures. Chilling damage to tubers occurs at temperatures below 38° F and appears as diffuse smoky grey areas inside the tubers. Blackening of the phloem, due to selective injury to this tissue, can also occur and closely resembles virus leafroll net necrosis. Tubers or portions of tubers that have been frozen at temperatures below 29° F are soft and watery. Frozen tissues tend to disintegrate and will eventually dry out. Symptoms of chilling and freezing can, but do not always, occur in the same tuber. Do not dig if a frozen crust is present on the ground. After a hard frost, discard any green tubers. Frost protection should be provided in storage and in transit.

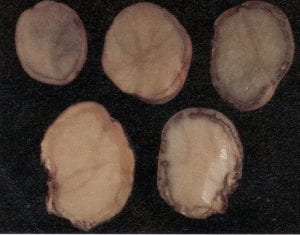
Fusarium dry rot – Fusarium spp. (fungus)
Dry rot is an appropriate description of this very common disease. Sunken and often shriveled areas on the surface of infected tubers are the most obvious symptom. When tubers are cut through the affected areas, tissues appear brown and collapsed, often with a white, pinkish, or yellow fungal growth, which may extend into the center of the tuber. Infection may occur anywhere on the tuber surface, including the stem end. Diagnosis of this disease can become complicated when secondary organisms, often soft rot bacteria, invade the infected tissue and a wet rot of the tuber results. Seed tubers infected with Fusarium spp. produce plants with reduced vigor and may result in poor stands. Infection can be minimized by avoiding tuber bruising during harvest and grading, and providing appropriate conditions for suberization (high humidity and good ventilation early in storage). Fungicide applications to seed before storage and at planting are also recommended.
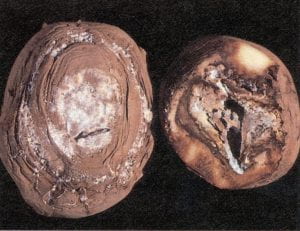
Late blight – Phytophthora infestans (oomycete)
Tubers can be infected during the growing season by spores washed from foliar lesions into soil to tubers or at harvest if tubers are dug before infected vines are thoroughly killed or while vines are still wet. Infected tubers may feel very firm to the touch. External tuber symptoms consist of patches of brown to purple discoloration on the skin, which become darker and sunken with time. When tubers are cut through these lesions, a reddish brown (mahogany), dry, firm rot is visible, progressing up to 1/2 inch deep into the cortex. These lesions have a somewhat granular appearance and spread unevenly into the tuber, particularly if the tubers have been stored for some time. Isolation of the causal agent may be necessary for an accurate diagnosis. This disease may be confused with pink eye, a disease not discussed here. Healthy tubers can be infected in storage if temperature and moisture are favorable. Invasion by soft rot bacteria often results in a wet rot. Tubers with evidence of late blight should be discarded before storing, and storages should be maintained at 36°–40° F with forced–air ventilation. Control in the field relies upon clean tubers, proper hilling, foliar fungicide applications, and proper vine–killing procedures. When the oomycete is present on the foliage, delay harvesting until vines have been dead for a minimum of 2 weeks. Cull piles and volunteer plants are important sources of inoculum and should be disposed of.

Leak – Pythium spp. (oomycete)
Infection by this common soil–inhabiting oomycete usually takes place at harvest through wounds or bruises, often during hot and (or) wet weather. External symptoms consist of gray to brownish lesions with a water–soaked appearance around wounds or near the stem end. A freshly cut affected tuber is grayish cream, but with exposure to air the tissue turns reddish tan, then brown, and finally black. A distinct dark brown margin may separate diseased from healthy tissue. Tuber flesh is usually granular, and frequently water is freely released upon cutting an infected tuber. Cavities may be formed in the flesh. Blackened tissue may resemble blackheart, but there is no watery exudate from blackheart. The cream–colored tissue bordered by a dark margin is suggestive of blackleg, but again, the firm tissue associated with leak is very different from the slimy rot that occurs with blackleg. To control this disease, avoid injury to tubers during harvest, especially under hot, dry conditions. Tubers should not be harvested until the periderm is mature. If this disease is detected in storage, the temperature should be maintained at 40°–45° F, and the tubers should be kept dry.
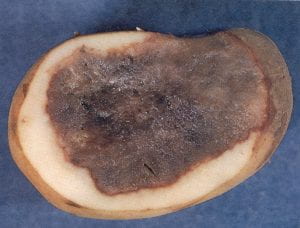
Mechanical injury and cracking (nonpathogenic)
Mechanical damage has repeatedly been singled out as a factor contributing to potato losses during the first 3 months of storage. Injuries provide an “avenue of entrance” for many pathogens in storage including Pythium leak, Fusarium tuber rot, and bacterial soft rot. Mechanical damage may consist of bruises, cuts, and deep abrasions that appear harmless externally, but internal examination often reveals the presence of infected tissue. “Harvest” or “thumbnail” cracks are small curved cracks that result from mild bruising and subsequent drying of tuber surfaces. Cracks may become apparent in marketing channels because of low relative humidity. These defects are usually shallow (up to 1/16 in. deep), but are sufficient to allow pathogen entry. They result from glancing impacts of tubers with other tubers or with harvesting and handling equipment; mechanical shock occurs in tubers with high turgor pressure. Controls for mechanical injury include gentle handling, harvesting only physiologically mature tubers, avoiding harvest from cold and wet soils, and using chemical vine killers to speed periderm maturation. Seed should be warmed before grading, or special care should be used in grading cold tubers.

Pink rot – Phytophthora erythroseptica (oomycete)
This disease is favored by cool weather and wet or poorly drained soils. External symptoms may be noted at the stem end or around eyes and lenticels. The infected area turns purplish to dark brown, with a black band between diseased and healthy tissues. Infected tubers are rubbery or spongy and may exude a watery liquid when squeezed, distinguishing this disease from blackheart. When tubers are cut, the infected tissue turns pink in a matter of minutes and then progressively darkens to brown and finally black. The tubers may have a pungent odor resembling formaldehyde. The pink coloration after cutting is useful in separating pink rot from Pythium leak. In addition, cavities do not develop in the flesh as with leak, and there is no dark border between diseased and healthy tissue on the inside of the tuber as occurs with blackleg and leak. The pathogen will spread in storage if the tubers are not kept dry. Because the pink rot organism is endemic in many soils, avoid planting in poorly drained areas of the field.

Ring rot – Corynebacterium sepedonicum (bacteria)
This is one of the most–feared potato diseases because the causal bacterium is easily disseminated and is responsible for serious market losses. Seed potato certification programs have a zero tolerance for this disease. When infected tubers are cut crosswise, the characteristic creamy yellow to brown breakdown of the vascular ring can be observed. The odorless decay is usually initiated near the stem end. Squeezing the tuber causes a cream–colored, cheesy exudate to ooze from the vascular ring, leaving a distinct separation of the vascular ring and surrounding tissue. In advanced disease stages, further tissue breakdown within and adjacent to the vascular ring by secondary organisms can cause external surface skin cracks, frequently accompanied by a reddish brown discoloration. In general, there is no spread from diseased to healthy tubers while in storage. Tuber symptoms are usually apparent at harvest, but some infected tubers remain symptomless for many weeks in cold storage. Infected seed and dried bacterial slime on crates, bags, cutting knives, storage bins, and machinery may all serve as sources of inoculum for ring rot. Unfortunately, automatic seed cutters and the picker–planter are ideal for spreading the disease. Certified seed and sanitation are of utmost importance for control.
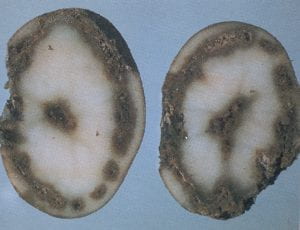
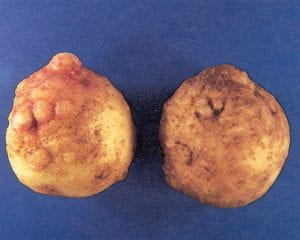
Root knot nematode – Meloidogyne spp. (nematode)
Root knot nematode is a relatively minor pest of potato in the Northeast. Infection of tubers by the root knot nematode often results in the formation of galls that appear as knobs or swellings on the tuber surface. These are somewhat similar in appearance to tuber malformation due to uneven growth. This symptom occurs when tubers are infected by relatively high nematode populations. If shallow cuts are made through the malformed tissues, the white swollen bodies of the female nematodes will be visible. Surrounding tissues will appear somewhat water soaked, and necrotic flecks may be present. However, when nematode populations are low, infection does not always result in swelling of the tuber surface, and the only symptom may be small necrotic flecks in the cortex of the tuber where the nematodes have penetrated. Identification of the species of root knot nematode can only be done through a nematode diagnostic service. Nematodes can survive in potato tubers and be a source of inoculum when infected tubers are used for seed.

Internal Diagnosis
Blackheart (nonpathogenic)
This injury occurs as the result of low oxygen levels in the interior of the tuber and is relatively easy to diagnose. The center of affected tubers is black to blue black, in an irregular pattern, and the border of the discolored area is usually very distinct. Darkened areas of the tuber are usually fairly firm, in contrast to those of tubers affected by Pythium leak, which are spongy. Affected tissues do not smell, and shrinking of the tissue may result in the formation of a cavity in the center of the tuber. Blackheart develops when tubers are held in a low–oxygen environment or when gas diffusion through the tubers is slowed because of extremely cold (32° F) or warm (96°–104° F) temperatures. This condition can also develop in the field when soils are flooded or in poorly aerated storages. Because seed–piece size is effectively reduced by the death of affected tissues, plant stand and vigor are likely to be reduced.

Black spot (nonpathogenic)
Black spot occurs in the tuber flesh just beneath the tuber periderm and appears as gray to black circular areas with diffuse borders. Because this injury is always the result of bruising, including pressure bruising, internal symptoms may be associated with flattening or other signs of mechanical injury on the tuber surface. However, black spot may occur in the absence of any obvious external damage, and mechanical injury to the tuber does not always result in internal symptoms. Many factors have been reported to affect the susceptibility of tubers to black spot. Tubers with low turgor pressure are most likely to be affected by black spot. The stem end is more susceptible than the apical end, and mature tubers are more susceptible than immature tubers. Seed quality is not appreciably reduced by black spot. Avoid deep storage piles, especially with more susceptible varieties.
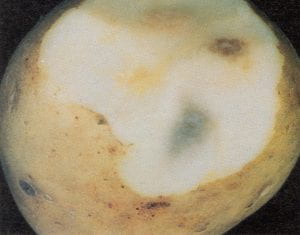
Fusarium wilt – Fusarium spp. (fungus)
Fusarium wilt can result in a variety of symptoms on tubers, ranging from surface decay to vascular discoloration. Several species of Fusaria can infect potato and cause wilt symptoms in the plant, and each causes slightly different symptoms on tubers. The most important of these pathogens can cause sunken brown necrotic areas at the stem attachment, firm brown circular lesions on the tuber surface, and brown discoloration of the vascular tissues. A shallow cut through the stem end reveals the streaky vascular discoloration referred to as “stem–end browning.” This disease may also cause a light brown to tan discoloration, extending a short distance on each side of the vascular system in the tuber, which may resemble symptoms of ring rot. However, this tissue is firm and does not produce a cheesy exudate when squeezed as does tissue affected by bacterial ring rot. Vascular discoloration resulting from net necrosis and vine killers may also be confused with Fusarium wilt. It is difficult to distinguish between Fusarium and Verticillium wilts based on tuber symptoms alone, and isolation of the causal pathogen is necessary for positive diagnosis. Infected tubers can be an important source of inoculum.

Net necrosis – Potato leafroll virus
Net necrosis of tubers is the condition resulting from current season infection with potato leafroll virus. A cut through the stem end of the tuber reveals a network of brownish black dots and streaks in the vascular ring and vascular (phloem) strands in the flesh. Often a second longitudinal cut should be made to show that the discolored strands continue throughout the tuber. The symptoms of net necrosis are most likely to be confused with frost necrosis and stem–end browning. Net necrosis differs from frost necrosis in that the dots and streaks of net necrosis are more clearly defined and occur in concentric rings. Stem–end browning, a phenomenon of unknown cause, consists of discolored strands, which do not extend more than 1/2 inch from the stem end. Controls include the use of certified seed, selection of varieties that fail to show necrosis, and the use of insecticides to control aphid populations, which transmit the virus.
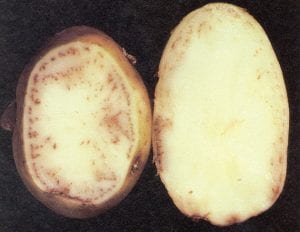
Verticillium wilt – Verticillium spp., (fungus)
This disease produces no external tuber symptoms, but causes a light brown discoloration in the vascular tissue of tubers, which may extend halfway through the tuber. Cavities sometimes develop inside tubers. Infected seed pieces often produce wilt–free plants, but soil adhering to tubers may contain the fungus and provide inoculum for infection of the subsequent crop. Pink eye, a pinkish or tan discoloration of the tuber surface around the eyes, may also develop on tubers produced from Verticillium–infected plants, but is caused by a bacterium, Pseudomonas fluorescens. Pink eye can be confused with the tuber rot phase of late blight. Because of the soilborne nature of Verticillium wilt, long rotations and the use of resistant potato varieties are recommended. Avoid late cultivation and hilling of susceptible varieties, for root pruning increases the risk of infection. The presence of the root lesion nematode, Pratylenchus penetrans, greatly increases yield losses due to Verticillium wilt.

More information/prepared by:
Originally created by Thomas A. Zitter and Rosemary Loria for Vegetable MD Online. Updated April 2021 by:
Margaret Tuttle McGrath
Associate Professor
Long Island Horticultural Research and Extension Center (LIHREC)
Plant Pathology and Plant-Microbe Biology Section
School of Integrative Plant Science
College of Agriculture and Life Sciences
Cornell University
mtm3@cornell.edu


