Carrot leaf blights are caused by two fungal pathogens, Alternaria dauci and Cercospora carotae and one bacterial pathogen, Xanthomonas campestris pv. carotae. Since any combination of the three pathogens may occur in a field, proper identification is important for employing the proper management strategies. For the two fungal pathogens, a 25% disease incidence level (25% of leaves examined show one or more lesions) is generally recommended to trigger the first fungicidal spray. Since bacterial leaf blight is an explosive disease that develops rapidly under hot, wet and windy conditions, the presence of a trace level in the field requires action. Yield loss due to these foliar diseases can be considerable, especially if they occur early in the season and are ineffectively managed (Figures 1 and 2). In addition, severe defoliation and weakened foliage results in reduced harvesting efficiency. Prior to the 2000 growing season, Alternaria leaf blight was the most prevalent in carrot fields in New York, but Cercospora and bacterial leaf blights have been more frequently observed since then. All three pathogens are known to be seedborne and are often found in and/or on seeds. Each can also infect and be harbored in wild carrot and Queen-Anne’s Lace (Daucus carota var. carota).


Symptoms and Signs
Alternaria leaf blight lesions are small and commonly found on the margins and tips of carrot leaflets. The lesions are irregular in shape and size, dark brown to black in color (Figures 3 and 3a). Under favorable conditions, the lesions become numerous and continue to expand until they ultimately coalesce giving the leaf tissue a blighted (burned) appearance. Eventually, the leaflets may shrivel and die. Large lesions can also develop on the petioles and may girdle and kill the leaves. Compared to Cercospora leaf blight, the lesions of Alternaria dauci are more evident initially on the lower, older leaves while the lesions of Cercospora carotae are more numerous on the younger as well as older leaves.


Cercospora leaf blight lesions are initially small necrotic flecks that develop into cream to gray colored lesions with dark colored definitive margins. These lesions are either circular in shape when in the interior of the leaf and more elongate along the leaf margin (Figure 4 and 4a). Like with Alternaria, as the lesions expand they can coalesce and lead to leaflet death. Petiole lesions are circular to elliptical in shape and have a lighter colored center.

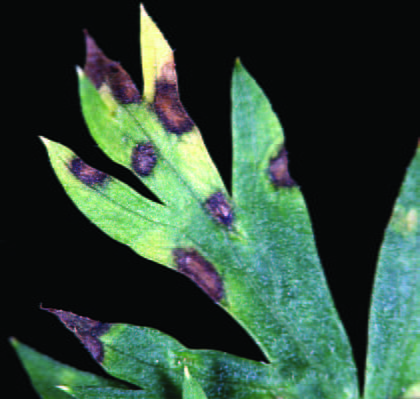
Bacterial blight lesions are initially small roughly circular lesions that are light brown to tan and shiny on the lower leaf surface, as if coated with varnish. As the infection progresses, the lesions become elongated in shape on the leaf blade, turn darker brown to black in color and develop water-soaked edges that are yellow and chlorotic (Figure 5 and 5a). On the leaf margin, the lesions are crescent shaped while on the carrot leaflets the lesions can appear more ‘V’ shaped. When the small leaf blades and the stems between the various sections of the carrot leaf are infected, they turn shiny brown and shrivel. The infection typically will progress down the main leaf and petiole veins; a characteristic that distinguishes it from Alternaria leaf blight. Very young, expanding leaves can be infected and the infections may result in leaf distortion and curving because the main stem has been stunted.


Disease Cycles
All three pathogens can be transmitted through infected or contaminated seeds and can survive on crop residue, which can act as a source of inoculum in subsequent cropping years. However, they cannot persist free in the soil for too long once the carrot tissues are thoroughly decomposed. The length of time the crop residue persists in soil is dependent on soil type and environmental conditions. The rate of spread of these diseases in the field is dependent on the initial level of inoculum available (seed contamination and/or infected residues), air temperature, and the presence of water from rain, irrigation, high humidity or dew.
Infection by Alternaria dauci is favored by moderate to warm temperatures and prolonged leaf wetness. Typically, as the temperature increases the duration of leaf wetness required for infection to occur decreases. Infection can occur in 8 to 12 hours at temperatures of 16-25 oC (61-77 oF). The fungus sporulates readily on dead necrotic tissue and the spores germinate readily in water droplets and dew.
Infection by Cercospora carotae requires a minimum of 12 hours of leaf wetness at 20-30 oC (68-86 oF). Under optimal environmental conditions, symptoms will develop in as little as 3 to 5 days. Due to the development of symptoms on younger tissue, this pathogen can be more devastating early in the season when there is limited foliage.
The bacteria, Xanthomonas campestris pv. carotae is dispersed from plant to plant by rain splash droplets or irrigation water as well as by insects, animals and machinery. The bacteria survive epiphytically on the surface of the leaves and disease symptoms develop once the population reaches a critical level. Optimal conditions for infection occur when the air temperature is 25-30 oC (61-86 oF) and the leaf surface is wet.
Management
An integrated pest management approach is necessary to effectively manage these diseases. This entails integrating and applying a variety of proactive management options which include planting clean seed, using resistant or more tolerant carrot varieties, rotating crops, and reducing plant stress through adequate nutrition and irrigation. In-season management decisions are then made based on field scouting to monitor disease development, the forecasted weather and utilizing thresholds to determine when the pathogen population has reached a level to cause economic damage.
Cultural management practices
One of the best ways to proactively reduce the severity of these three leaf blight diseases is to use pathogen-free and treated seeds in order to reduce or eliminate this potential inoculum source.
Resistant varieties are also important. Several carrot varieties resistant to at least one of these diseases are now commercially available. Bolero is one of few resistant to all three diseases. Additionally, repeated observations and variety evaluations in New York and elsewhere have documented that commercial varieties not bred to be resistant differ greatly in their susceptibility to these blights. Carson, Ithaca and Calgary were found to be less susceptible to Alternaria leaf blight, while Neal, Bergen and Bristol were found less susceptible to Cercospora leaf blight.
Practices that contribute to reducing leaf wetness duration and soil moisture (wider row spacing, breaking compacted layer, and planting on raised ridges) will be beneficial. In addition, Alternaria leaf blight is generally more severe on poorly fertilized and stressed carrots. Therefore, keeping carrots vigorously growing (proper fertility, gibberellic acid application) and free from injury will aid in the control of Alternaria leaf blight.
Harvesting carrots on time will contribute to reduced crop loss as a result of the leaf blight diseases. Immediately plowing under the crop debris will reduce inoculum build-up and survival of leaf blight pathogens. Also, it is important to practice a minimum of a 2-year rotation out of carrots to allow time for decomposition of crop residue and also the reduction of fungal and bacterial leaf blight pathogens and their diseases.
Fungicides
For labeled fungicides see current Cornell Integrated Crop and Pest Management Guidelines for Commercial Vegetable Production.
Research conducted in Canada established 25% disease incidence as a threshold for timing the first fungicide application for managing Alternaria and Cercospora leaf blights. Field data collected over several growing seasons in New York has verified and validated the use of the 25% disease incidence as the trigger for the first fungicide application under New York growing conditions. Due to differences in variety susceptibility, it is important to scout and make management decisions by variety, since different varieties will reach the disease threshold level at different times.
Once the first fungicide application is made, subsequent sprays may be determined based on: 1) a calendar spray schedule, based on recommended intervals for the specific fungicide used; 2) by continuing scouting to monitor disease progress and monitoring temperature and forecasted rainfall (an application is needed if disease severity has increased, rainfall is predicted for the following 5 days and/or night temperature is >60 oF) or 3) by using the Tom-Cast model (Tomato early blight model) which is available through the Network for Environment and Weather Awareness (NEWA). The Tom-Cast model takes into account temperature and leaf wetness to calculate a disease severity value (DSV). It is recommended that a fungicide application be made once >15 DSV’s have accumulated since the previous application. Once a fungicide application is made the accumulated DSV’s go back to zero and the process begins again. For more information about NEWA and Tom-Cast contact your county extension educator or NEWA at 315-787-2206 or on the web at https://dev.newa.cornell.edu/.
For bacterial blight, as soon as the first symptoms are observed in the field, a copper-based bactericide should be applied. If not managed with the first onset of symptoms, bacterial leaf blight will progress rapidly under hot, wet and windy conditions. Research results have indicated that the application of a copper-based bactericide may delay the need for the next fungicide application to manage Alternaria and/or Cercospora leaf blights. Careful monitoring of disease incidence and severity as well as weather conditions will reduce the number of sprays needed to manage blight diseases, thus enabling the grower to reduce input costs as well as reduce environmental impact.
Alternation of fungicides is recommended to prevent resistance development and reduce costs. Research results have also suggested that alternating one of the registered fungicides with a copper material was also effective in managing Alternaria and Cercospora and may prove beneficial in keeping bacterial leaf blight under control.
Scouting/Field Monitoring
As stated previously, it is not only important to scout carrot fields by variety due to differences in disease susceptibility, but also to sample enough plants to provide an accurate assessment of the level of disease on that variety. While walking across a field in a ‘V’- or ‘W’- shaped transect (Figure 6), a minimum of ten sampling stops should be made (more stops should be considered in larger fields).
Five leaves from five adjacent plants are scored for disease incidence. A leaf is infected if one or more lesions are observed. The 25% disease incidence threshold for Alternaria and Cercospora to trigger the first fungicide application means that 25% of the 50 leaves sampled (approx. 12 leaves) are showing symptoms either of Alternaria or Cercospora leaf blights. Disease severity is determined on a scale of 1 to 9 and based on the percentage of the leaf surface blighted. A scale of 1 = 0% (healthy), 2 = up to 1%, 3 = 2-5%, 4 = 6-10%, 5 = 11-20%, 6 = 21-30%, 7 = 31-40%, 8 = 41-50% and 9 = over 50% of the leaf surface blighted. Disease severity is used to determine disea

se progress and along with forecasted rainfall and temperatures, the need for additional fungicide applications.
More information/prepared by:
2004 NYSIPM program factsheet by B.K. Gugino, J. Carroll, J. Chen, J. Ludwig, and G. Abawi. Photos by J. Ogrodnick. Updated June 2021 by
Margaret Tuttle McGrath
Associate Professor
Long Island Horticultural Research and Extension Center (LIHREC)
Plant Pathology and Plant-Microbe Biology Section
School of Integrative Plant Science
College of Agriculture and Life Sciences
Cornell University
mtm3@cornell.edu
Acknowledgements
We would like to specially thank Carol MacNeil (Senior Extension Educator: Ontario, Wayne, Yates and Steuben Counties), Arlie McFaul (Extension Educator, Lake Plains Region), Tim Widmer (Former post-doctoral fellow; Department of Plant Pathology, NYSAES, Geneva) and collaborating carrot growers (Larry Christensen, Ed Hansen, Dale Hemminger, John Williams, Peter Call/ My-T-Acres Farms, R.B. Glaizer/ L-Brooke Farms and Gary Kludt) for their contributions to the research, demonstration and/or documentation of results presented in this summary. Also the financial support received from the RAMP/USDA, NYS/Cornell IPM, and the NAPIAP programs are gratefully acknowledged. Produced by the New York State Integrated Pest Management Program, which is funded through Cornell University, Cornell Cooperative Extension, the NYS Department of Agriculture and Markets, the NYS Department of Environmental Conservation, and USDA-CSREES. Design by Media Services, Cornell University. Layout by Karen English, New York State IPM Program.


