Black dot disease of potato, caused by the fungus Colletotrichum coccodes, is generally considered to be a weak root pathogen of potato. Recent studies in New York and elsewhere have revealed, however, that this disease must be considered as part of the total disease complex affecting potato.
Although not as serious a tuber- or soilborne pathogen as black scurf (Rhizoctonia solani), silver scurf (Helminthosporium solani), or common scab (Streptomyces scabies), Colletotrichum can cause severe rotting of below-ground plant parts and early plant decline leading to discolored tubers and reduced yields. The same black dot organism causes anthracnose or ripe-fruit disease of tomato, and can occur on other solanaceous crops and weed species.
Symptoms and signs
The name “black dot” accurately describes the numerous dot-like, black sclerotia that can appear on tubers, stolons, roots, and stems both above and below ground level. A significant portion of the stem may be covered with sclerotia (Fig. 1) which are easily seen after vine kill. Total root growth is reduced and appears brown to black in color (Fig.2). Sclerotial bodies can be found on both roots and stolons. Some of the roots and stolons appear white in this photograph because the cortical tissue has been sloughed off due to severe infection. Apparently, enzymes secreted by the pathogen are responsible for the foot and root rot stages. Small, brownish lesions caused by Colletotrichum, bearing a resemblance to Rhizoctonia-induced lesions, may appear on recently infected stolons.
Tuber infection appears as brownish to gray discoloration over a large portion of the tuber (Fig. 3), or as roundish spots often larger than 1/4-inch in diameter. Silver scurf can also appear as patchy discoloration on the tuber surface, but takes on a silvery sheen when the affected area is moistened. Both pathogens frequently occur on the same tuber (as seen in Fig. 3, where a silver scurf-infected area appears just below the largest sprout).
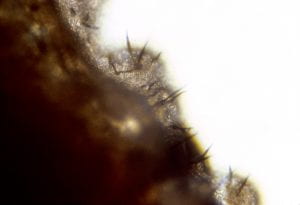
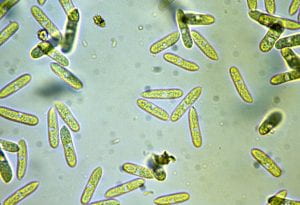
Closer examination of the same tuber reveals the presence of dot-like sclerotia of Colletotrichum within the discolored area (Fig. 4). The sclerotia can easily be seen with a hand lens. Sclerotia develop into clumps (acervuli) on tubers and affected parts following storage or after overwintering in the field. Acervuli produce characteristic dark, needle-shaped spines, or setae (Fig. 5), and give rise to spores (conidia) (Fig. 6) which are responsible for new infections. On infected tubers, the organism does not penetrate intact tuber skin, but can grow and sporulate on damaged tissue. This limited growth, however, does not appear to provide entry sites for secondary fungal or bacterial invaders in stored tubers.




In a three-month greenhouse test to study the effects of black dot on tuber infection and yield, disease-free plantlets of three varieties (Chippewa, Hampton, and Green Mountain) were inoculated with Colletotrichum spores at the time of transplanting to sterile potting soil. The varieties chosen represent early to late maturities. The relative productivity of the late variety Green Mountain was most greatly affected. Similar results following inoculations with Colletotrichum have been reported from Idaho. In the same greenhouse experiment, bacterial soft rot was unexpectedly found on tubers inoculated with.black dot. The highest incidence occurred in the variety Hampton, followed by Chippewa; soft rot was negligible for Green Mountain. The same trends in the number of tubers with soft rot occurred when pots were inoculated with Rhizoctonia, but the incidence was at much lower levels. These results suggest that decay of roots and stolons of greenhouse- and field-grown plants may create a favorable environment for soft rotting bacteria, and may increase the chance for infection of especially susceptible varieties.
Although foliar symptoms of black dot have not been reported in eastern United States, they have been observed in Idaho and bear a resemblance to early blight. The yellowing and wilting of foliage can also be confused with wilts caused by Verticillium and Fusarium spp.
Epidemiology
The disease cycle for black dot is straightforward. The fungus overwinters as sclerotia either on tuber surfaces or on plant debris in the field (potato, tomato, and other hosts). Although not an active soil inhabitant, the fungus appears to survive there for long periods. The importance of soilborne versus tuberborne inoculum is not known.
In the spring, sclerotia on plant debris or on tubers develop into acervuli and then give rise to spores. Infection of below-ground plant parts probably continues throughout the season, especially when plants are under stress. Poor soil drainage and low plant fertility increase disease incidence. Discoloration can occur on all tuber sizes, including tubers as small as 1/2-inch in diameter. Foliar symptoms, although not common, have been reported in western states, and may contribute to premature death of potatoes and increased colonization of daughter tubers.
Control
- Because black dot is both soil- and tuberborne, it is important to use long rotations (3-4 years) and clean tubers for planting. Deep plowing will bury infected debris and encourage decomposition. Do not include solanaceous crops in the rotation scheme; be sure that solanaceous weeds are controlled.
-
No resistant varieties are available, but late-maturing varieties are more vulnerable to yield reduction. Varieties that appear to be moderately resistant include Eva, Genesee, Keuka Gold, Lehigh, NorDonna, Norland, and Norwis, Varieties that are moderately susceptible to susceptible include Andover, Banana, Chieftain, Monona, Pike, Reba, Superior, and Yukon Gold. Avoid plant stress and maintain adequate fertility. Select well-drained land if possible.
-
Several fungicides are labeled. See current Cornell Integrated Crop and Pest Management Guidelines for Commercial Vegetable Production.
More information/prepared by:
Originally created by Thomas A. Zitter, Louis Hsu, and Donald E. Halseth (1989) for Vegetable MD Online. Updated April 2021 by:
Margaret Tuttle McGrath
Associate Professor Emeritus
Long Island Horticultural Research and Extension Center (LIHREC)
Plant Pathology and Plant-Microbe Biology Section
School of Integrative Plant Science
College of Agriculture and Life Sciences
Cornell University
mtm3@cornell.edu


