Printer-friendly .pdf version of this page.
Healthy roots are essential for vigorous plant growth and productivity because they mine the soil for nutrients and water, especially when the environmental conditions are stressful such as during a drought. One of the major constraints to root health is the damage caused by fungal and nematode infection. Simple soil-indexing procedures are useful tools for determining the capacity of soil to suppress soilborne fungal and nematode pathogens. These types of tools can be used to establish baseline measurements and identify fields that may or may not be of concern. Since the soil is a dynamic medium and as a result responds to inputs and changes in soil management practices, it is not surprising that soilborne pathogens also respond to these changes. So subsequent monitoring of the impact of selected management practices using soil- indexing bioassays is important for determining the effectiveness of the selected management practices and can also help lead to managing root diseases on an as-needed basis (IPM strategy).
Soil Sampling for Soilborne Pathogen Assessment
Collection of a soil sample that represents the area of interest or sampling unit is critical when assessing for soil pathogens. Soilborne pathogens are not uniformly distributed in the soil; they tend to be patchy and uneven. To account for this variability, composite soil samples are typically collected in an ‘X’, ‘V’ or ‘W’-shaped pattern in the field or area of interest. It is also important to collect soil from within the rooting depth of the crop where the highest pathogen populations tend to be concentrated, near an available food source. Once collected, the composite soil sample, consisting of ten to twelve sub-samples, should be mixed thoroughly and 2-liters placed in a labeled plastic bag. It is important to keep in mind that you are going to be extrapolating the soil bioassay results to make a larger field-scale management decision.
Once collected, proper storage of the soil sample is also critical. Overheating or freezing of the sample can reduce the pathogen population and lead to an underestimation in pathogen pressure. It is recommended that soil samples are kept out of direct sunlight and are stored in a cool location (~ 40F) until the bioassays are conducted.
The timing of when the soil samples are collected is also important for interpretation of the results. The pathogen population tends to be the highest at or shortly after the harvest of a susceptible crop and after harvest there tends to be more time available for sample collection. However, keep in mind that it is the pathogen population at the time of planting that will, in part, determine damage on a susceptible crop during the season.
Soil Bioassay with Bean for Soilborne Fungal Pathogens in Vegetable Cropping Systems
Snap beans are susceptible to and a good indicator for, the predominant soilborne fungal pathogens present in vegetable and forage cropping systems in New York and the Northeast. These pathogens including Rhizoctonia solani, Thielaviopsis basicola, Pythium spp., and
Fusarium spp. are responsible for diseases such as pocket rot, black root rot as well as Fusarium and Pythium root rots on crops including carrot, beet, pea, tomato, cabbage, and bean, etc.
To set-up the bioassay, the composite soil sample (consisting of at least 2 L of soil) is divided between four 4-in. pots or other suitable container (milk carton, plastic cup, etc. with drainage holes) and planted with 4 to 6 snap bean seeds cv. ‘Hystyle’ or some other susceptible cultivar. The seeds are treated with a combination of fungicides to prevent seed decay and seedling diseases since we are interested in rating the roots themselves. The pots are maintained in a greenhouse or under a bank of lights in a barn or outside if the weather permits, watered regularly and fertilized once a week (NPK 20:20:20) or as needed. After four to five weeks the roots are knocked out of the pots/containers, the bulk soil shaken off and the roots washed under running water or in a bucket of water.
The hypocotyl and roots are then rated for root health on a scale of 1 to 9. A rating of 1 = white and coarse textured hypocotyl and roots; healthy, 3 = light discoloration and no necrotic lesions to a maximum of 10% of the hypocotyl and root tissues with lesions, 5 = approx. 25% of hypocotyl and root tissue have lesions, but the tissues remain firm to the touch and there is little decay to the root system and a rating of 7 to 9 = 50 to ≥ 75% of the hypocotyl and roots are severely symptomatic and at advanced stages of decay (Figure 1). Roots with ratings below ≤ 3.0 are considered good, healthy and functional while, roots rated ≥ 7.0 are considered poor and have a greatly reduced ability to translocate water and nutrients to above portions of the plant.

Soil Bioassays with Lettuce and Soybean for Root-Knot and Lesion Nematode Pathogens
The northern root-knot nematode (Meloidogyne hapla) and root-lesion nematode (Pratylenchus penetrans) are the two primary plant-parasitic nematode pathogens impacting the quantity and quality of vegetables in New York and the Northeast. It is the initial soil infestation densities at planting that will determine the severity of damage and potential yield losses, if any. Thus, monitoring soil populations using soil indexing bioassays will facilitate managing them on an as-needed basis and also enable the efficacy of the management practices to be assessed.
Lettuce is an excellent bioassay plant for assessing Northern root-knot nematode infestation levels. Large and distinct galls develop on the roots that can easily be quantified by counting the number of galls on the root system. Soybean works well for lesion nematode because the narrow chocolate-brown to black lesions that develop on the roots after infection can be observed against the white roots using a hand magnifying lens.
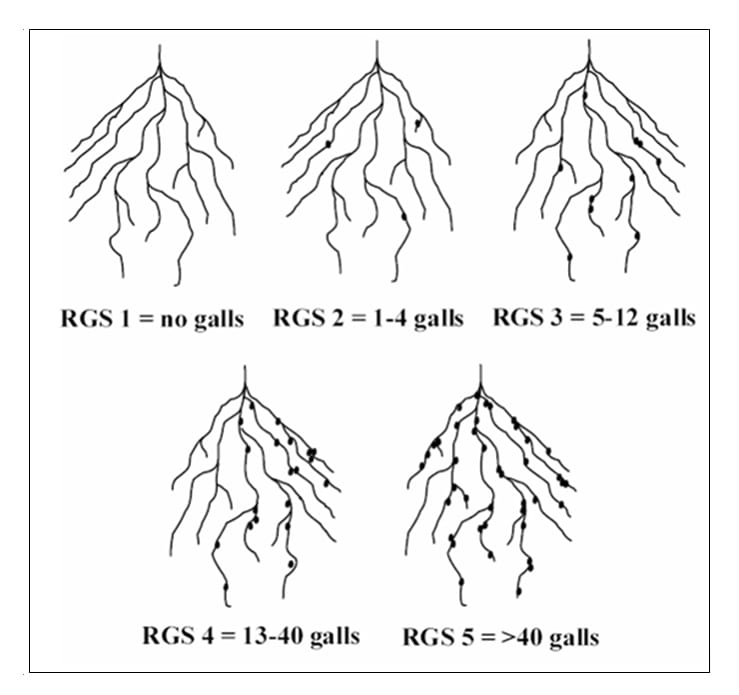
Similar to the soil bioassay with bean, the thoroughly mixed composite soil sample is divided into 2 to 4 pots or other suitable container and planted with either 2 lettuce or 2 soybean seeds (or small seedlings). Although we use the lettuce cv. ‘Ithaca’, resistance to Northern root-knot nematode is not available so any commercially available cultivar will work. To-date, the soybean cultivar ‘SG1405 RR’ has been used in the evaluation of the protocol however, other cultivars are also suitable, as all the soybean cultivars evaluated in our greenhouse tests have been found to be susceptible to lesion nematode. The pots are maintained as described previously and the roots are evaluated after 4 weeks for lettuce and after 2 to 3 weeks for the soybean bioassay. Again, the roots are knocked out of the pots and the lettuce roots are rated for root-galling by counting the number of galls on the entire root system and then using the following root-galling severity (RGS) scale: RGS 1 = no galls, 2 = 1-4 galls, 3 = 5-12 galls, 4 = 13-40 galls and 5 = >40 galls (Figure 2). If planting carrot, a very susceptible crop, use a RGS ≥ 2.0, for onion a RGS ≥ 3.0 and for less sensitive crops like potato and bean, a RGS ≥ 4.0.

brown lesions.
The number of lesions that develop on the soybean roots is a reflection of the lesion nematode infestation level in the soil. It can be roughly estimated by counting the total number of elongated chocolate to dark brown lesions observed on the main taproot (the side roots may be removed prior to counting). A hand lens and supplemental light may improve lesion visibility and thus a more accurate evaluation. A soil population of 100 lesion nematodes per 100cc soil is damaging to onion and several other vegetable crops, especially when they are stressed due to biotic and/or abiotic factors. Results of soil bioassays conducted under greenhouse conditions have suggested that this same density of lesion nematode is associated with the observation of approx. 15 (range 10 to 20) diagnostic lesions on the taproot of the soybean bioassay plant. Observing more or less lesions on the taproot will suggest < 100 or > 100 lesion nematodes per 100cc soil. It is advised that management options be implemented when 100 or more lesion nematodes per 100cc soil are predicted.
Data related to the use of these bioassays in addition to information regarding select practices for managing soilborne pathogens will be presented.
Prepared by/more information:
Beth K. Gugino (now at Penn State) and George S. Abawi (professor emeritus)
For more information, contact:
Beth Krueger Gugino
Professor in Vegetable Crop Pathology
Plant Pathology and Environmental Microbiology
College of Agricultural Sciences
The Pennsylvania State University
219 Buckhout Laboratory
University Park, PA 16802-4507
bkgugino@psu.edu


